KAMPALA, UGANDA: Eloipharm (U) Ltd, the manufacturers of Elo-Enjo, a sexual-arousing herbal medicine have clarified claims made by the National Drug Authority (NDA) over its name and approval to the Ugandan market.
NDA yesterday raised an alarming red flag accusing Eloipharm, the maker of the medicine of rushing the product to the market despite having had the initial name of the product rejected by the authority.
However, speaking to DailyExpress on Friday morning, Dr Stephen Lutoti, the proprietor of Eloipharm and a Senior Pharmacist and lecturer at Makerere University says the product is duly approved by NDA only that the authority changed its initial name to avoid confusing the product.
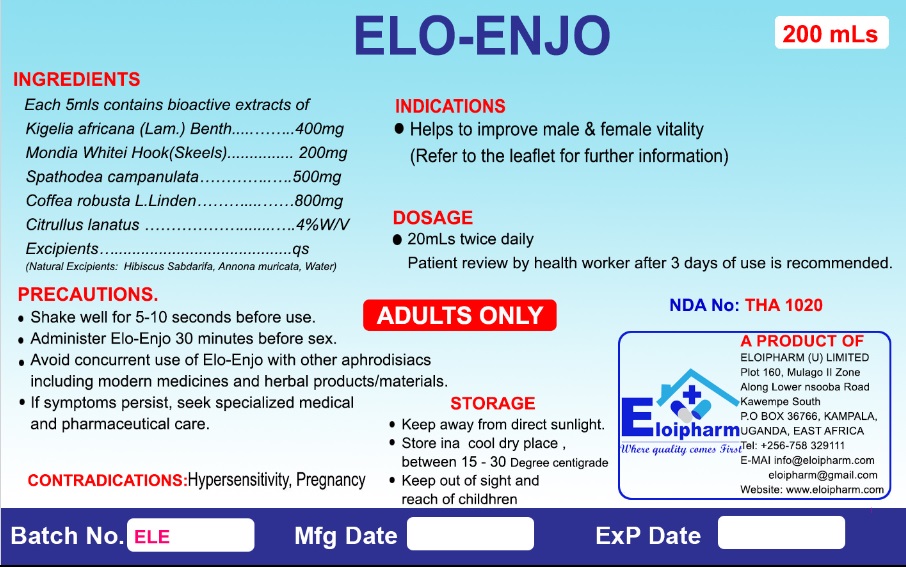
“This is to clarify that the product in the media is approved by National Drug Authority ( NDA) and is available in pharmacies and drug shops across Uganda,” the scientist told DailyExpress.
Dr Lutoti says NDA requested for change of name claiming it has the word enjoy and that the public may take it as a beverage yet it is for medical purposes.
“Eloipharm as the manufacturer reluctantly allowed the change of name by dropping the y at the end. NDA Drug register has ELO-ENJO instead of ELO-ENJOY. We have since implemented the label change in compliance,” Dr Lutoti explained.
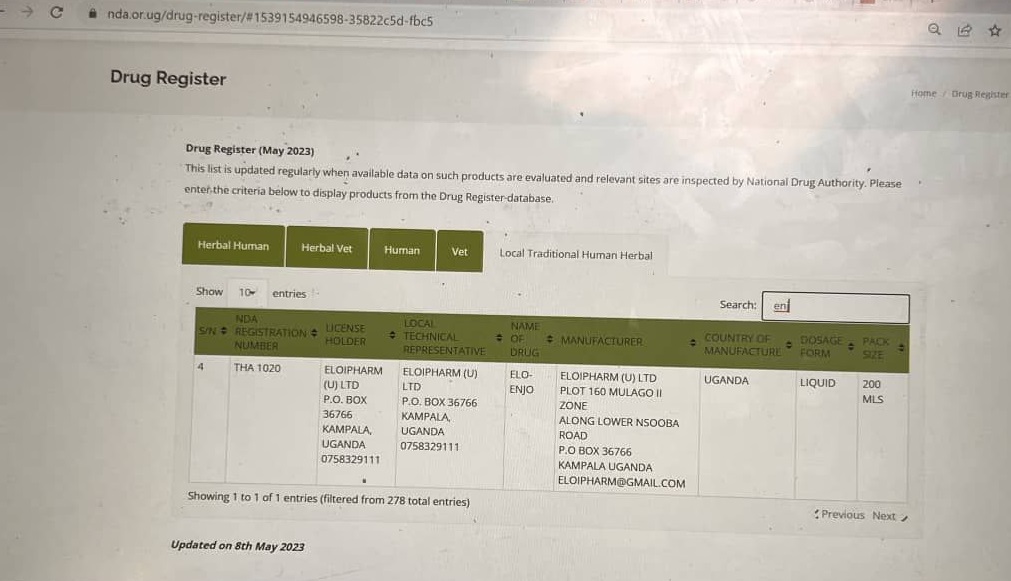
The NDA Register which was last updated on 8th May 2023 shows the local traditional herbal drug was approved by the authority under the product name “Elo-Enjo” instead of “Elo-Enjoy” which the maker had proposed.
Established under the National Drug Policy and Authority (NDP/A) Act, Cap. 206, the core mandate of NDA is to ensure the availability, at all times, of essential, efficacious and cost-effective drugs to the entire population of Uganda as a means of providing satisfactory healthcare and safeguarding the appropriate use of drugs.
For more inquiries about Elo-Enjo Herbal Medicine, you may call +256758329111 or WhatsApp Dr Stephen Lutoti on +256 782764180 ; email: eloipharm@gmail.com
Do you have a story or an opinion to share? Email us on: dailyexpressug@gmail.com Or join the Daily Express WhatsApp channel for all the latest news and trends or join the Telegram Channel for the latest updates.


